

Join Seneca to get 250+ free exam board specfic A Level, GCSE, KS3 & KS2 online courses. Want to learn more about Reactivity Series?
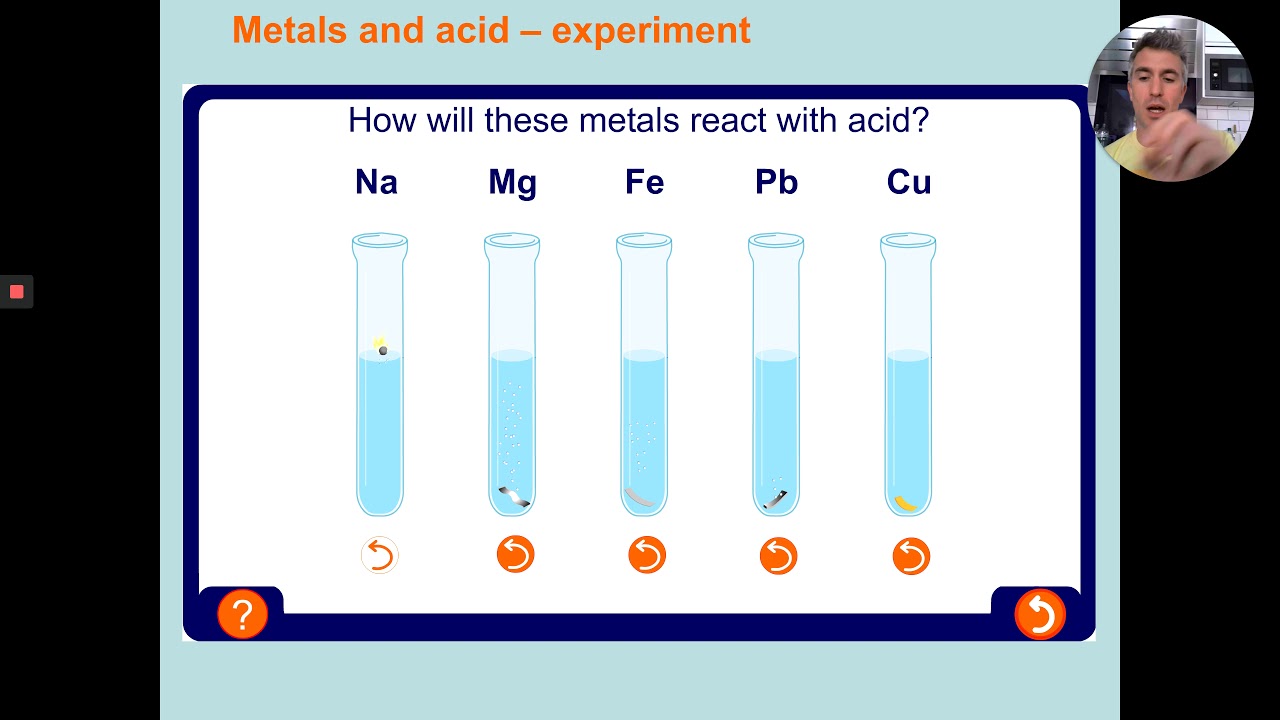

Because of this, the metal has to be extracted from the ore (rock) where the metal compound is found. Most metals are only found as compounds because the metal has reacted with other elements in the past. Most metals react with dilute acids to produce a salt and hydrogen gas. Potassium, sodium and lithium all react quickly with cold water to produce a metal hydroxide and hydrogen gas. Platinum, however, is less reactive than copper and so cannot displace copper from a copper sulfate solution. Magnesium + copper sulfate → magnesium sulfate + copper Magnesium is more reactive than copper, so magnesium can displace copper from a copper sulfate solution to create magnesium sulfate. A displacement reaction happens when a more reactive metal (one that forms positive ions more easily) displaces a less reactive metal from a compound. This is called a displacement reaction.Ī metal can only displace another metal from a compound if it is located above it in the reactivity series. These are called native metals.Ī more reactive metal (one that forms positive ions more easily) can displace a less reactive metal from a compound. For example, both magnesium and zinc can react with hydrogen ions to displace H 2 from a solution by the reactions: Mg (s) + 2 H + (aq) H 2 (g) + Mg 2+ (aq) Zn (s) + 2 H + (aq) H 2 (g. The top metals are more reactive than the metals on the bottom. Very unreactive metals, such as gold and platinum, are found in the Earth’s crust as pure metals. The activity series is a chart of metals listed in order of declining relative reactivity. Metals can be arranged in order of their reactivity. The easier it is for a metal to form its positive ion, the more reactive the metal is. This is the reactivity series, from most reactive to least reactive (and a. When metals react with other substances, the metal atoms always form positive ions. Only metals above hydrogen will react with water/steam and hydrochloric acid. You can watch the YouTube Video below to learn how to memorise the list of metals in the Reactivity Series.Ĭlick on the following link for the video on O-Level Chemistry. So sodium and potassium, for example, are extremely.

Do look out for it! YouTube Video Tutorial on The Reactivity Series of Metals The reactivity series lists the metals (and a couple of non-metals) in order of decreasing reactivity. We will discuss this in more detail in the next blog post. The Reactivity Series is actually determined by scientists through the reactions of a list of common metals with water, steam and dilute hydrochloric acid. Note that the layer of Al 2O 3(s) has been removed before experiments were conducted by scientists to determine the position of aluminium in the Reactivity Series of Metals. As such, aluminium is not very reactive in our everyday life because it is covered (and thus protected) by the layer of Al 2O 3(s). * Aluminium tends to oxidise readily in air to form aluminium oxide, Al 2O 3(s). You can download your copy of the Reactivity Series of Metals. The reactivity series also includes a list of reactive metals with potassium at the top being very reactive and platinum at the bottom which doesnt. Else, just use the one that is presented here. In fact, you can come out with your own memorising method if you want. In Singapore, whether you are taking the GCE O-Level Pure Chemistry syllabus or any of the IP Chemistry syllabus, you will need to remember the sequence of metals in the Reactivity Series.Īs such, we use mnemonics (super memory techniques) to help our Sec 3 and 4 O-Level Pure Chemistry and IP Chemistry Tuition Class students to recall the list of metals. This is simply due to the fact that zinc is more reactive than gold and thus it will react faster with air. For example, the Reactivity Series will show you immediately why coins made of zinc metal look dull after some time while silver and gold coins remain bright and shiny for long period of time. This is useful because it allows you to predict how a particular metal will undergo a certain chemical reaction. Metals are listed in the Reactivity Series from the most reactive to the least reactive. Today, we shall take a look at The Reactivity Series of Metals. In the previous blog post, we have discussed on the physical properties of pure metals as well as the four main reasons why we use metals in the form of alloys.


 0 kommentar(er)
0 kommentar(er)
